| Page 1
Page 2 Page 3 Page 4 Page 5 Page 6 Page 7 Page 8 Page 9 Page 10 Page 11 |
|
|
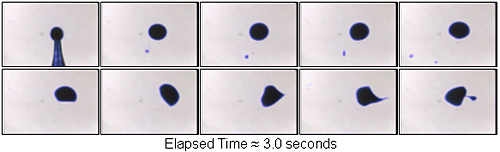
| Alcohol At Lower Temperature
Relative To Water Alcohol drops at a lower
temperature relative to the water they are placed on exhibit peculiar
behavior. This series of pictures illustrates a drop that stays
almost completely whole for a period of 3.0 seconds. This is in
contrast to the room temperature drop dissolving on room temperature
water that breaks up in 1 to 2 seconds at most.
|